The newly discovered pathway for formaldehyde oxidation may be an important general mechanism in tropospheric chemistry. In the new pathway, absorption of sunlight allows organic molecules to react with atmospheric oxygen, a reaction not previously observed. According to the researchers behind the findings, many compounds in the atmosphere can undergo this process, especially at low altitudes.
’We discovered a new way in which molecules in the atmosphere react,’ he says Scott Cable at the University of New South Wales, Australia. In this process, known as photophysical oxidation (PPO), a molecule absorbs sunlight and reacts with atmospheric oxygen to form free radicals before it breaks into fragments, he explains. In a common photochemical oxidation (PCO) reaction that has been known for decades, the molecules are first split by sunlight, and then the fragments react with oxygen. „Importantly, the free radical fragments formed in the first stage of PCO can be measured individually in the atmosphere or in the laboratory,” Gable points out.
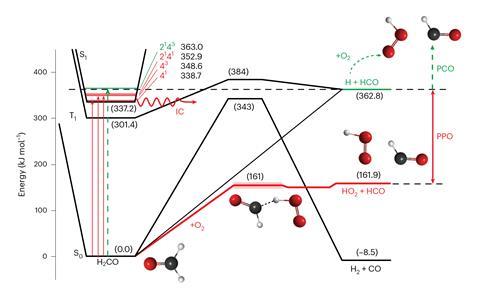
The team demonstrated the PPO mechanism using formaldehyde as a model system. Meredith Jordan The University of Sydney notes that many organic compounds released into the environment are oxidized to carbon dioxide to form formaldehyde. „But most importantly for our research, the spectra and photochemistry of this compound are well understood,” he says. 'Without this extensive prior knowledge, we cannot find the source of the PPO.’
Normally, when formaldehyde absorbs sunlight at wavelengths below 330nm it splits into formyl and hydrogen radicals, which then react with oxygen to form hydroperoxyl radicals (HO).2・). In the new PPO reaction, light excites an electron in the molecule without breaking it. 'This electronic excitation energy can then be converted into vibrational energy, which we also call heat,’ explains Cable. 'Typically, the „hot” molecules cool back to room temperature by colliding with other species in the atmosphere, mostly nitrogen. What we discovered is that if the collision partner is oxygen, which makes up about 21% of the atmosphere, the process of collisional cooling also leads to the reaction.’
The researchers irradiated formaldehyde molecules with a laser beam in the presence of oxygen and used IR spectroscopy to analyze the products formed. 'At laser energies too high to break the C-H bond, no PCO should take place, and we found PCO-like molecules in the IR spectrum,’ says Cable. But the reactions involved were very fast, so while the team could detect the end products, they couldn’t see the free radical fragments that formed.
To address this issue, they jointly performed additional cavity ring-down spectroscopy experiments Christa Fitzen from the University of Lille, France. 'This is a very specialized system, designed to detect HO2・The radical is an intermediate produced in the BPO reaction,’ says Cable. „We measured the HO level2By changing the absorption wavelength, we again observed that this species is produced at wavelengths where the CH bond of formaldehyde should not be broken.
’Calculations showed us that the energy limits for BPO are half of those for PCO,’ says Jordan. 'Breaking the C-H bond in formaldehyde requires twice as much energy as the reaction of hot formaldehyde with oxygen. In other words, although PCO reactions were halted in our experiments, there was plenty of potential for PCO to occur. Using kinetic modeling, the team demonstrated that BPO is fast enough to compete with the collisional cooling process. As the reaction rate increases with oxygen pressure, PPO becomes more important at lower altitudes.
’Understanding the Pathways of Extremists—Especially HOX The species formed in the atmosphere is critical to our understanding of how pollutants are processed and the subsequent generation of other molecules thought to be involved in aerosol formation. Rebecca Caravan at Argonne National Laboratory in the US, which was not involved in the project. This work really highlights the important connection between chemical physics and atmospheric chemistry, and it will be very exciting to learn more about the general nature of PPO and its atmospheric implications.’

„Oddany rozwiązywacz problemów. Przyjazny hipsterom praktykant bekonu. Miłośnik kawy. Nieuleczalny introwertyk. Student.