Researchers at Article In collaboration with researchers from Osaka University, Tokyo Polytechnic University, Kyushu University and National Tsing Hua University, they developed a method to introduce alkali metals into graphene interlayers. A sheet of carbon atoms arranged in a hexagonal lattice is called graphene. The atomic structure of doped alkali metal atoms, a hexagonal close-packed bilayer structure, was successfully observed by them.
The performance of rechargeable batteries has a significant impact on the driving range of electric vehicles and smartphone usage time. It is possible to improve the performance of these electronic gadgets if rechargeable batteries can accumulate more electrical power.
Graphite, the electrode material used in batteries, is composed of multiple layers of graphene with alkali metals between the layers to enable electron flow during charge and discharge. Achieving a higher density of alkali metal deposition between graphene layers can improve electrical efficiency.
X-ray and electron diffraction experiments last century made it generally known that graphene interlayers could only hold a single layer of alkali metal. The theoretical charging limit is the number of monolayer alkali metal atoms that fill each layer to capacity.
However, there are no studies that directly observe the atomic structure of intercalated alkali metals to confirm whether graphene layers can hold only a single layer of alkali metal atoms, or whether other methods can create multilayers or high-density alkali metals.
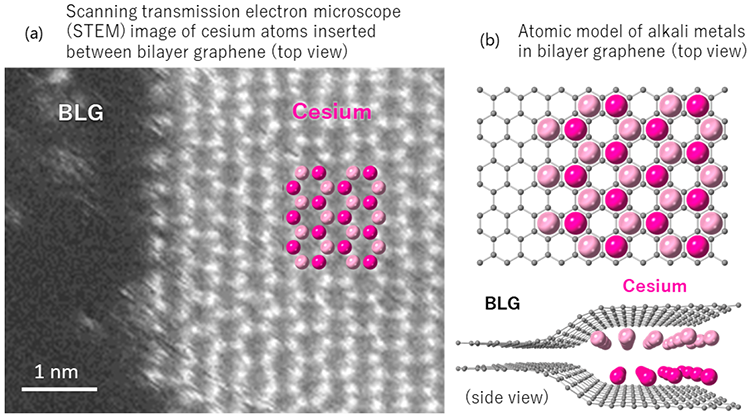
Researchers have devised a method to introduce dense alkali metals between layers of graphene. Using high-performance low-voltage (60 kV) electron microscopy, they have successfully detected the arrangement of alkali metal atoms between graphene layers.
Due to their flexible interstitial spacing, alkali metals are densely packed into a two-layer structure of surface layer graphite and bilayer graphene. This allows twice as much alkali metals to be injected. To increase the capacity of secondary alkaline ion batteries, graphene with two layers of alkali metal insertion is expected to act as an electrode material.
Background
Japan is making significant strategic investments in developing rechargeable batteries for use in electric cars and information technology.
Reducing battery weight and increasing battery capacity are among the fundamental technologies needed to improve the performance of rechargeable batteries. Carbon layers form graphite, a strong and lightweight electrode material.
Lithium and other alkali metal ions undergo electron transfers throughout the charging and discharging process. Increasing the amount of alkali metal ions at the electrodes is essential to increase the capacity of rechargeable batteries.
Nevertheless, atomic-level observations of electrode structure have proven difficult to accomplish using conventional measurement and evaluation. Furthermore, there are very few design methods to efficiently introduce alkali metal ions into graphene layers.

„Oddany rozwiązywacz problemów. Przyjazny hipsterom praktykant bekonu. Miłośnik kawy. Nieuleczalny introwertyk. Student.
