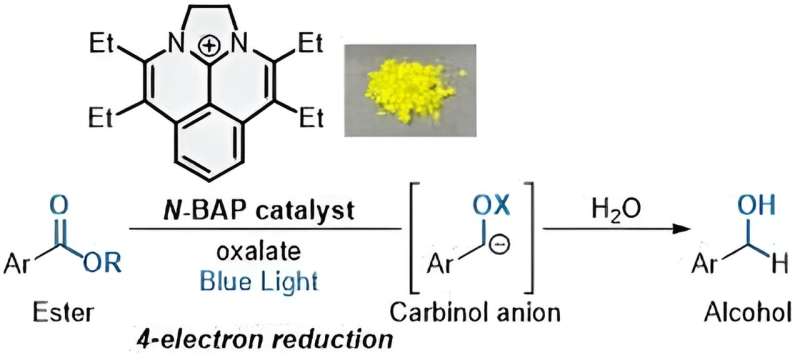
Multielectron reduction of esters photocatalyzed by N-BAP. Credit: Shintaro Okumura
The sweet smell of strawberries and other fruits is thanks to a chemical compound called an ester, which is also found in many fats and polyesters. The ubiquitous compound can be broken down to produce alcohols and other chemicals desirable for use across industries, including pharmaceuticals and cosmetics, but the process can be financially and environmentally costly.
Now, a team of researchers from the National Institutes of Natural Sciences (NINS) in Japan has developed a new approach using light as an energy source. They published their findings on June 14 Journal of the American Chemical Society.
In a seemingly counterintuitive move, to break down or reduce, chemical barley-esters, scientists actually add electrons to the compound. The addition of electrons causes the ester-containing groups to be reduced to more basic components. Conventional methods for ester reduction have high levels of reactive and difficult to handle metal reductants.
Now, researchers are exploring the use of sustainable photocatalysts. Photocatalysts or catalysts are known to catalyze an electron transfer process between the catalyst and organic compounds that activate when excited by light.
Conventional photocatalysts, consisting of expensive and non-renewable noble metals, reduce limited organic compounds, and typically add only one electron to the compounds. Called single-electron transfer (SET), the process must continue several times until the desired number of electrons is added to achieve the target reduction of esters.
„In the past decade, photocatalytic reactions have received significant attention as preferred methods relevant to the United Nations Sustainable Development Goals (SDGs) in organic synthesis,” said Shintaro Okumura, assistant professor at the Institute for Molecular Sciences (IMS). ) of NINS.
„Photocatalysts catalyze redox reactions using visible light as an energy source in the absence of metal reductants. However, photocatalytic reactions with multi-electron transfer processes are less developed, so the photocatalytic reduction of esters to produce alcohols that require four electrons remains underdeveloped. The photocatalytic reduction of esters to produce alcohols is a formidable challenge because it An unprecedented four-fold continuous SET process is required,” Okumura said.
To achieve this quadruple SET process, the researchers developed a new photocatalyst named „N-BAP”. When irradiated with blue light, the photocatalyst initiates a reaction to form one chemical group that reacts with water and a second carbon-based chemical group. By adding oxalate, a negatively charged molecule widely found in nature, the reaction can add four electrons in rapid succession—resulting in the desired alcohols.
„Coupling the N-PAP catalyst with oxalate as a trace reductant allows a rapid sequence of four-electron reduction of esters to form carbinol ions, with subsequent protonation yielding alcohols,” Okumura said.
„This work can lead to new transformation of esters and is expected to contribute to a sustainable society as a green organic synthesis relevant to the SDGs.”
More information:
Shintaro Okumura et al., Multi-Electron Reduction of Esters by a Diasabenzazenaphthenium Photoredox Catalyst, Journal of the American Chemical Society (2024) DOI: 10.1021/jacs.4c05272
Quotation: Novel photocatalyst enables efficient ester reduction by blue light (2024, June 15)
This document is subject to copyright. No part may be reproduced without written permission except for any reasonable manipulation for personal study or research purposes. Content is provided for informational purposes only.

„Oddany rozwiązywacz problemów. Przyjazny hipsterom praktykant bekonu. Miłośnik kawy. Nieuleczalny introwertyk. Student.
