Characteristic of B.KK channels in murine and human BCCs. debt: eLife (2024) DOI: 10.7554/eLife.92511.3
Researchers have revealed how a channel that regulates cellular potassium levels alters metabolism in breast cancer cells, promoting tumor growth.
study, Published Inside eLifeIt was described by the authors as providing convincing evidence that an intracellular subpopulation of a specific potassium channel reprograms breast cancer cells toward the Warburg phenotype, one of the metabolic hallmarks of cancer.
Ion channels are intracellular gateways that tightly control the inward and outward flow of essential ions such as potassium and calcium, and are thought to be associated with cancer malignancy and progression.
Cancer cells show increased energy requirements due to their high growth rates, and can shift their metabolism from a process that requires oxygen to a process that allows them to grow in an oxygen- and nutrient-poor environment – a process known as the Warburg effect.
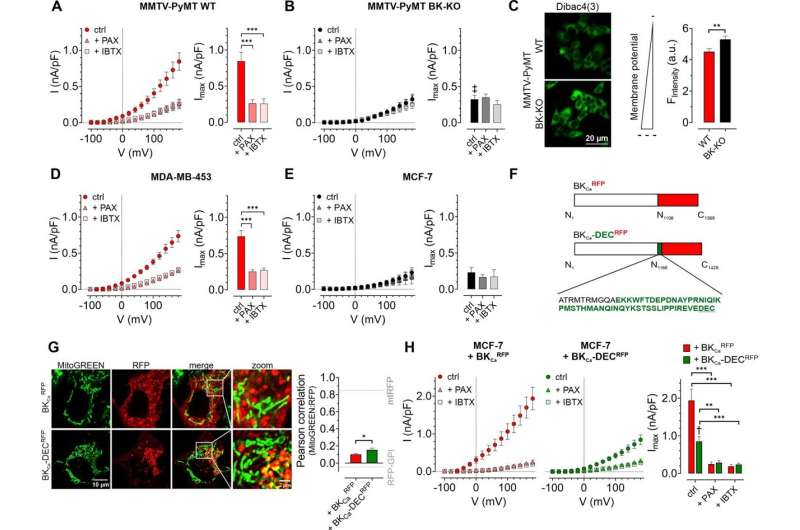
Researchers from Lukowski’s laboratory at the University of Tübingen, Germany, together with international/national collaboration partners, have now demonstrated that these metabolic processes rely on ions such as potassium and calcium, and that their dynamics are controlled by the „large-conductor Ca”.2+ Implemented Q+ channel”—or BKCa. This channel has previously been reported to promote breast cancer progression, but the exact mechanism underlying this effect is unknown.
To close this gap in knowledge, a multidisciplinary research team used BKCa-proficient and -deficient human cancer cell lines and cancer cells from BKCa-deficient mice to study the role of different BKCa subpopulations in cancer cell metabolism and growth. They found that breast cancer cells lacking BKCa had lower intracellular calcium levels. Furthermore, this change in calcium levels occurred not only in the main body of the cell, but also within substructures such as mitochondria – the tiny power plants that cells use to produce energy.
Based on these results, the team investigated whether BKCa affected metabolic processes in breast cancer cells. As they expected, cells with BKCa had an increased release of lactate and lower oxygen consumption than breast cancer cells without BKCa, suggesting a metabolism mediated by this type of ion channel.
To investigate this further, the authors looked at how different energy sources are used in mitochondria when BKCa is activated. Cells can build using energy in the form of a molecule called ATP. When BKCa is present in mitochondria, cells prefer to consume ATP rather than generate it. Furthermore, this switch causes hydrogen peroxide levels to increase, which causes further genetic damage and contributes to cancer progression.
Finding that BKCa helps cancer cells reprogram their metabolism, Dr. Bischoff, the study’s first author, looked for evidence of the Warburg effect by measuring lactate concentrations over time; High levels of lactate secretion indicate a shift toward a metabolism that does not utilize oxygen.
As expected, the presence of BKCa not only increased lactate secretion, but also increased the ability of breast cancer cells to grow without oxygen. Together, these results indicate a significant tumor-promoting effect of mitochondrial BKCa in breast cancer.
Finally, Bischop et al. investigated whether mitochondrial BKCa is present in primary human-derived breast cancer tissues. They found that of the 551 tissue samples that tested positive, 10 showed significantly higher expression of BKCa.
„Our experiments highlight the presence of an ion channel within the energy production machinery in breast cancer cells, which promotes profound bioenergetic changes and ultimately stimulates the growth of breast cancer cells in an oxygen-depleted environment, such as that seen in a solid tumor.” concludes senior author Robert Lukowski, professor of experimental pharmacology at the University of Tübingen.
„Taken together, the expression of mitochondrial BKCa in breast cancer patients may be used as a prognostic or therapeutic marker and presents a new anti-cancer therapeutic strategy.”
More information:
Helmut Bischoff et al., mitoBKCa is functionally expressed in murine and human breast cancer cells and contributes to metabolic reprogramming, eLife (2024) DOI: 10.7554/eLife.92511.3
Quotation: Scientists reveal how a potassium ion channel reprograms energy production in cancer cells (2024, June 4)
This document is subject to copyright. No part may be reproduced without written permission except for any reasonable manipulation for the purpose of personal study or research. Content is provided for informational purposes only.

„Oddany rozwiązywacz problemów. Przyjazny hipsterom praktykant bekonu. Miłośnik kawy. Nieuleczalny introwertyk. Student.
